Methylamine Oxidation by Methylotrophic bacteria
Back to main science page
Back to My Research pages
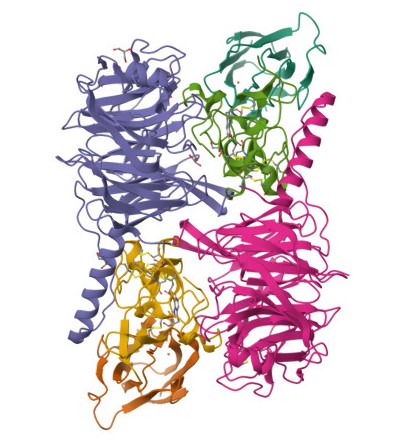
The image shows Methylamine dehydrogenase combined with its electron aceptor amicyanin (Pearson, A.R., Pahl, R., Davidson, V.L., Wilmot, C.M. 2007; taken from the Protein database)
Our publications about methylamine oxidation and Blue copper proteins in methylorophs
Lawton, S.A. and Anthony, C. (1985). The roles of cytochromes and blue copper proteins in the oxidation of methanol and methylamine in organism 4025, an obligate methylotroph. Journal of General Microbiology 131, 2165-2171. PDF
Lawton, S.A. and Anthony, C. (1985). The role of blue copper proteins in the oxidation of methylamine by an obligate methylotroph. Biochemical Journal 228, 719-726. PDF
Auton, K.A. and Anthony, C. (1989). The 'methylamine oxidase' system of an obligate methylotroph. Biochemical Journal 260, 75-79. PDF
Auton, K.A. and Anthony, C. (1989). The role of cytochromes and blue copper proteins in growth of an obligate methylotroph on methanol and methylamine. Journal of General Microbiology 135, 1923-1931. PDF
Anthony, C. (1990). Blue copper proteins involved in methanol and methylamine oxidation. Methods in Enzymology 188, 284-289. PDF
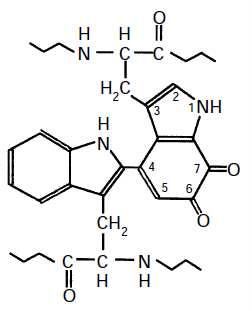
Many of the methylotrophs that gow on methanl are also able to grow on methylamine. The methylamine dehydrogenase (MNDH) responsible for the first step in its oxidation was first described by Peter Large (with Rod Quayle) in Methylobacterium extorquens in 1961. The assay system was almost the same as that which we had described for the methanol dhydrogenase (MDH) in the same organism. Its novel prosthetic group was later shown to be an orthoquinone called Tryptophan Tryptophylquinone (TTP). So methylamine dehydrogenase is, like MDH, a quinoprotein.
A is the artificial electron acceptor phenazine methosulphate. In the cell it is amicyanin (see below).
During the oxidation of methylamine the 6,7 orthoquinone is reduced to an orthoquinol. The hydrogens are then transferred to phenazine methosulphate in the enzyme assay system. In whole cells the acceptor is amicyanin which accepts the electrons, the hydrogens being released as protons.
Electron transport during methylamine oxidation
Tobari and Harada (1981, 1984) showed that the natural electron acceptor for MNDH in Methylobacterium is a specific blue copper protein which they called Amicyanin. It is similar to the well known Azurin (also involved in electron transport in this organism) but has this special funcition.
Our work used an obligate methylotoph that grew only on methanol or methlamine. It was unusual in that after growth on methylamine (but not on methanol) suspensions of harvested bacteria were a remarkable blue colour.
As in all methylotrophs there ae two periplasmic c-type cytochromes; cytochrome cH (donor to the cytochrome oxidase) and Cytochrome cL (electron acceptor for methanol dehydrogenase). For full description click here.
My studemt Ashley Lawton purified the two blue copper proteins and the two cytochromes c from Organism 4025 and showed that they were all located in the periplam and that they could all interact with one another.
During growth on methylamine a high concentration of copper was required and large amounts of blue copper protein were produced (35 times more than in Methylobacterium). Amicyanin was 94% of the blue copper protein. This facilitated the study of electron trasport during methylamine oxidation.
A remarkable observation was that the concentration of cytochrome cH, the typical electon donor to the cytochorme oxidase, was much lower during growth on methylamine than it was during growth on methanol.
When a suspension of cells with methylamine was was allowed to become reduced its colour was pink but when shaken to oxidise electron transport components it was blue. This is because cytochromes are only bright red when reduced, and blue copper proteins are only blue when oxidised.
My student Kevin Auton
purified the membrane oxidase from organism 4025. This was a cytochrome co as found in another obligate methylotroph Methylphilus methylotrophus. Its usual electron donor during methanol oxidation is cytochrome cH . However, when grown in high copper concentrations the concentration of azurin is 5x higher than cytochrome cH. In reconstituted 'oxidase systems' containing pure blue copper proteins and the oxidase it was shown that azurin is an excellent electron donor to the oxidase.
In optimal conditions during growth on methanol:
Methanol is oxidised by way of MDH, cytochrome cL, cytochrome cH and the oxidase.
In optimal conditions during growth on methylamine:
Methylamine is oxidised by way of MNDH, amicyanin, azurin and the oxidase.
Summary of electron transport pathways during oxidation of methanol and methylamine
These were demonstrated using all the various combinations of dehydrogenases, cytochromes and blue copper proteins. It is noteworthy that either cytochrome cH or azurin could be the electron donor to the oxidase whichever dehydrogenase was involved.
As shown here these pathways lead to to a proton gradient plus an electrochemical gradient (the proton motive force) which drives ATP synthesis catalysed by a membrane ATP synthase.